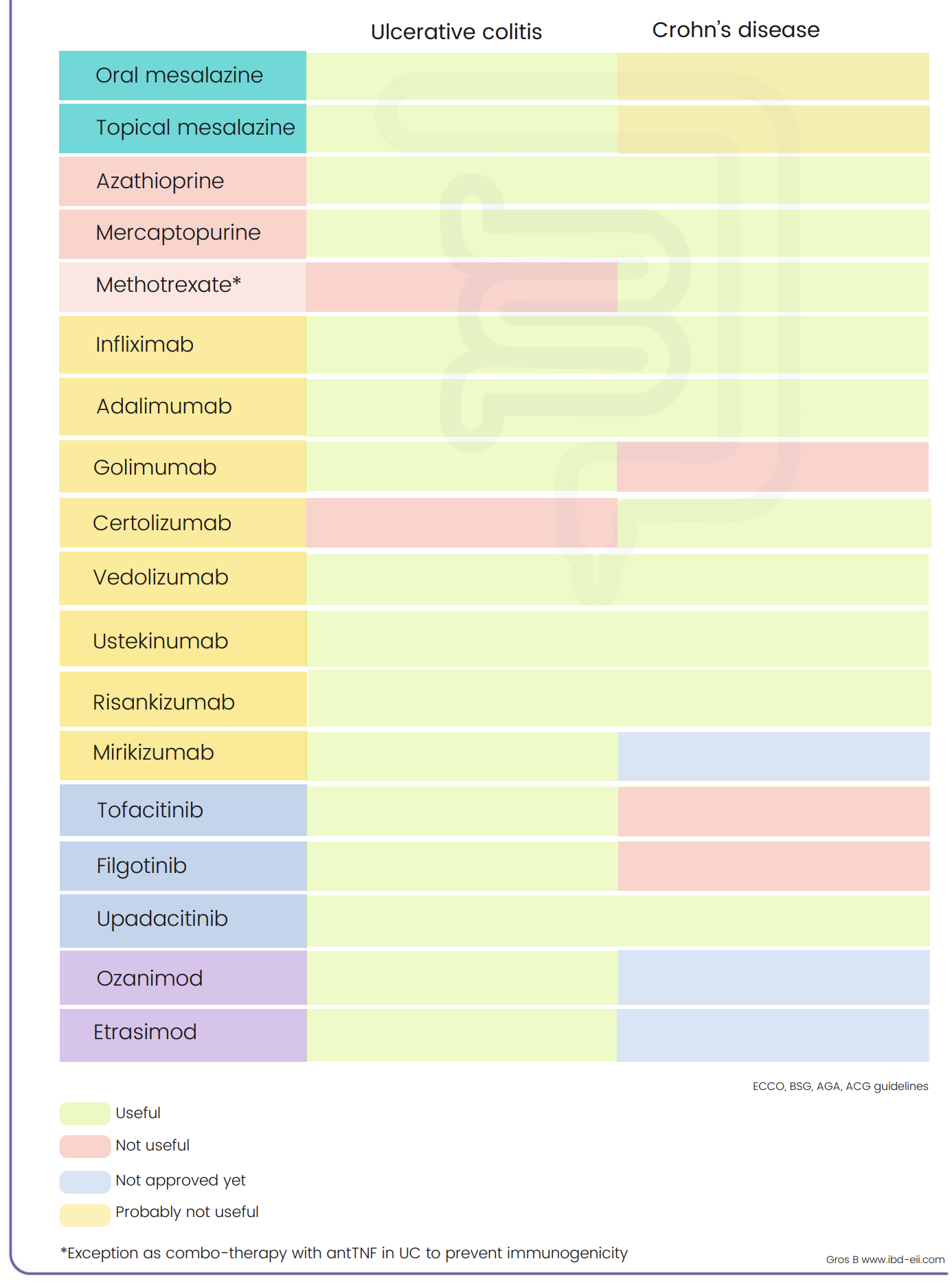
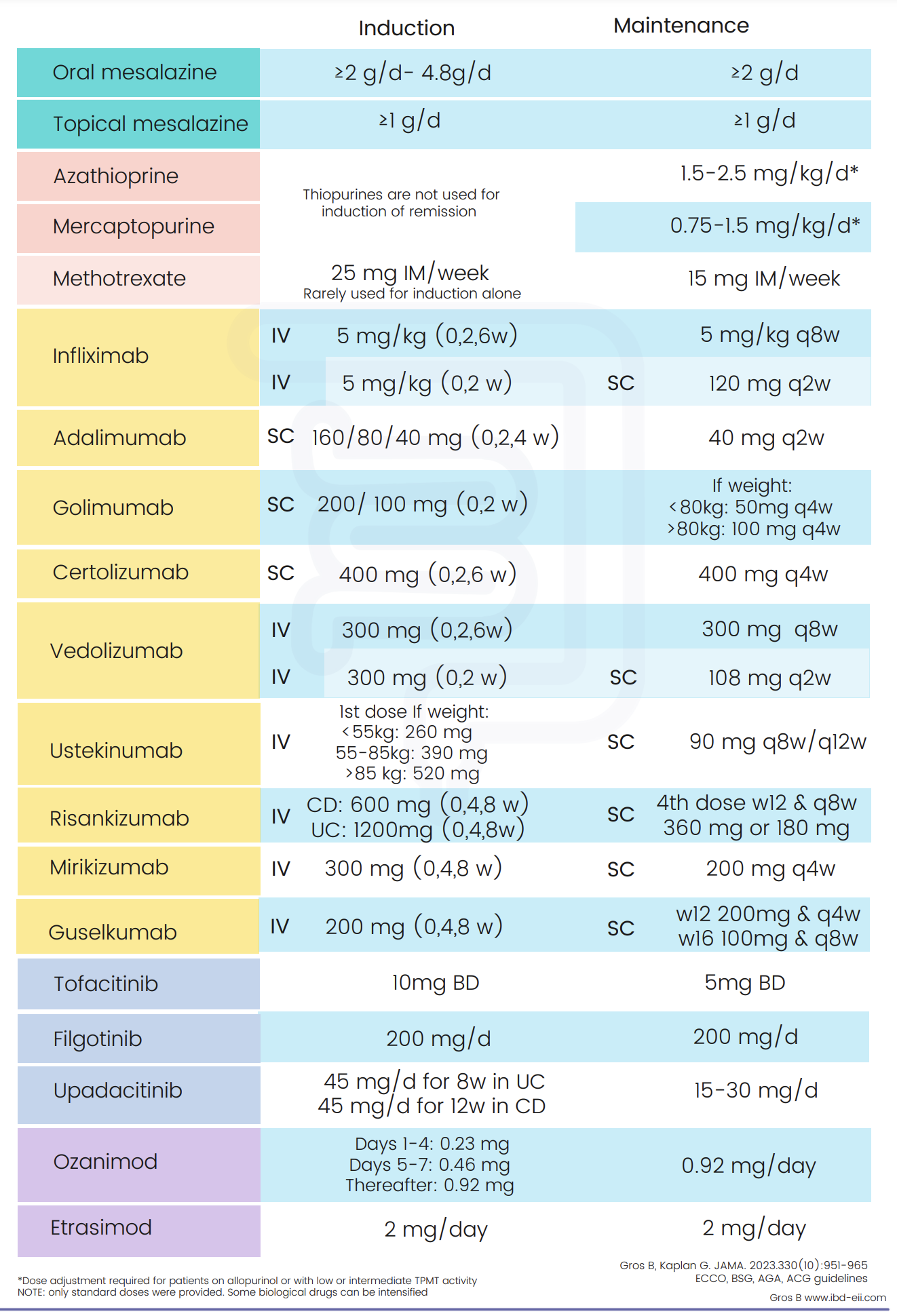
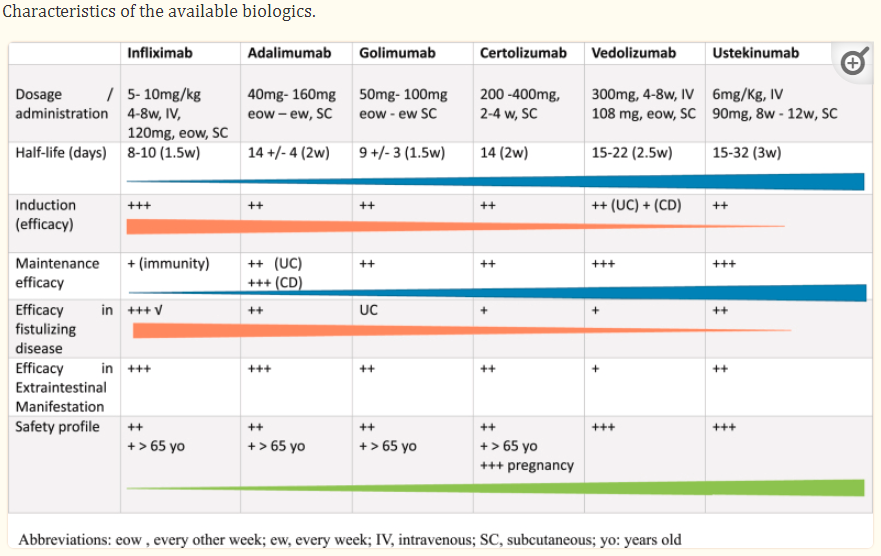
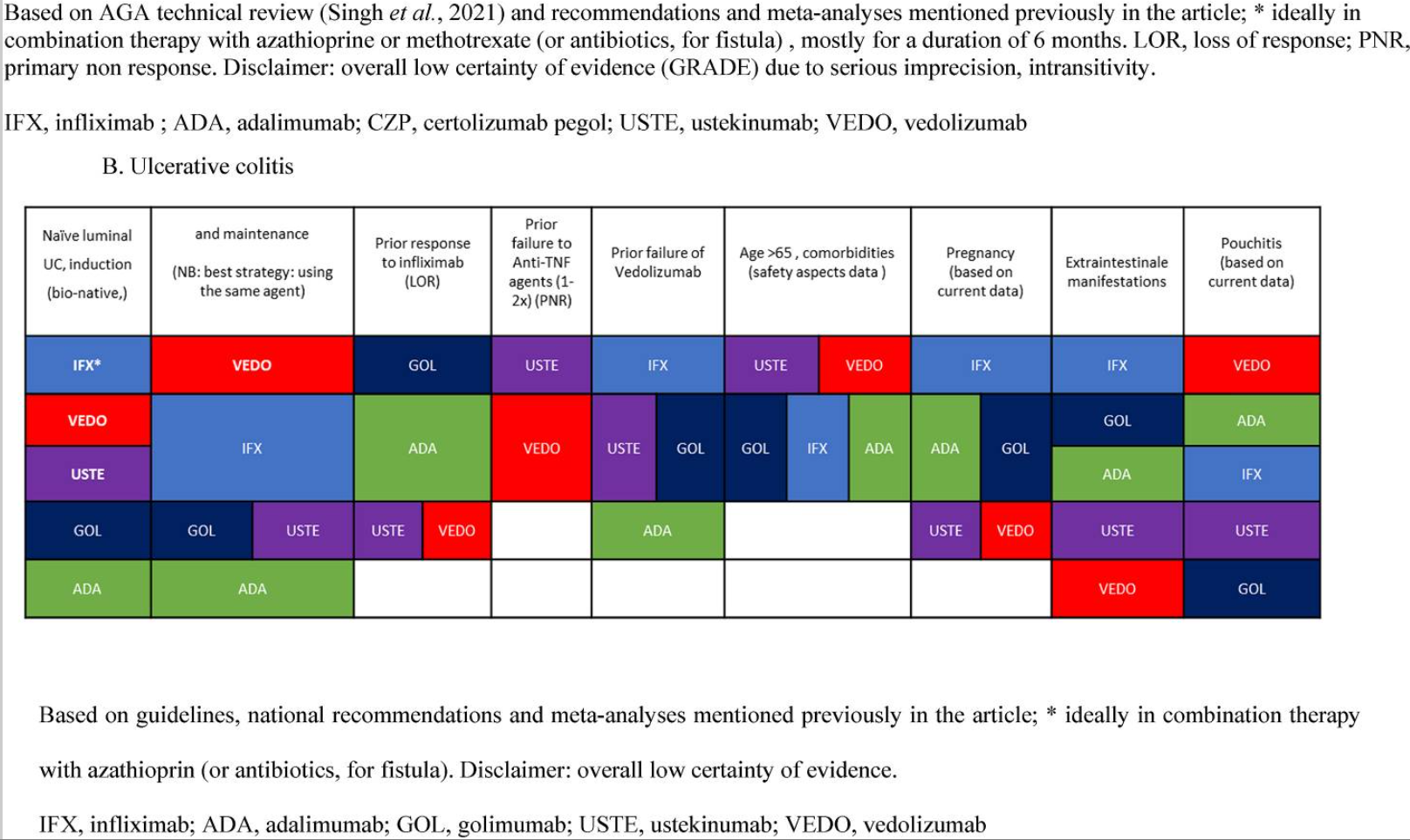
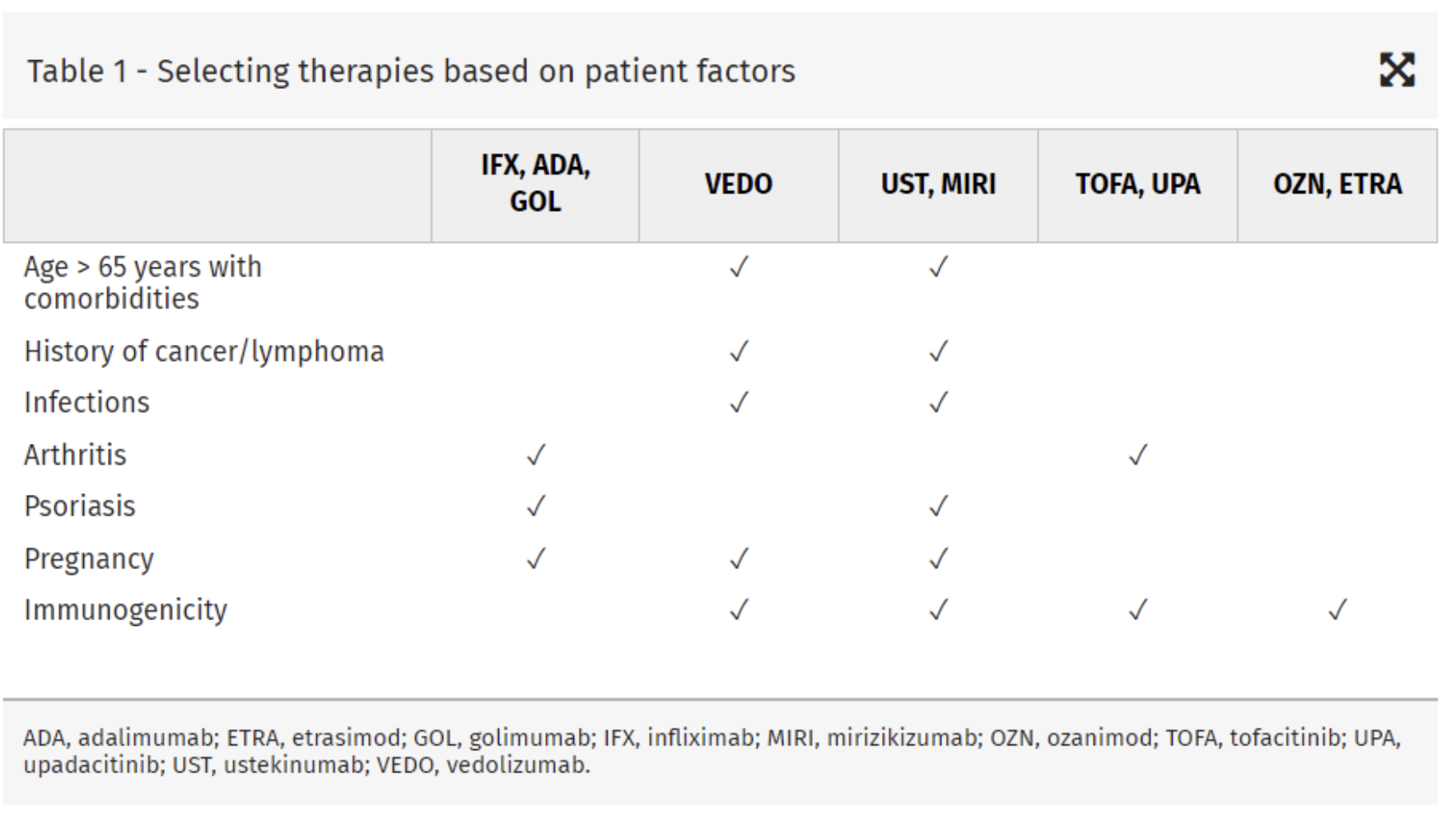
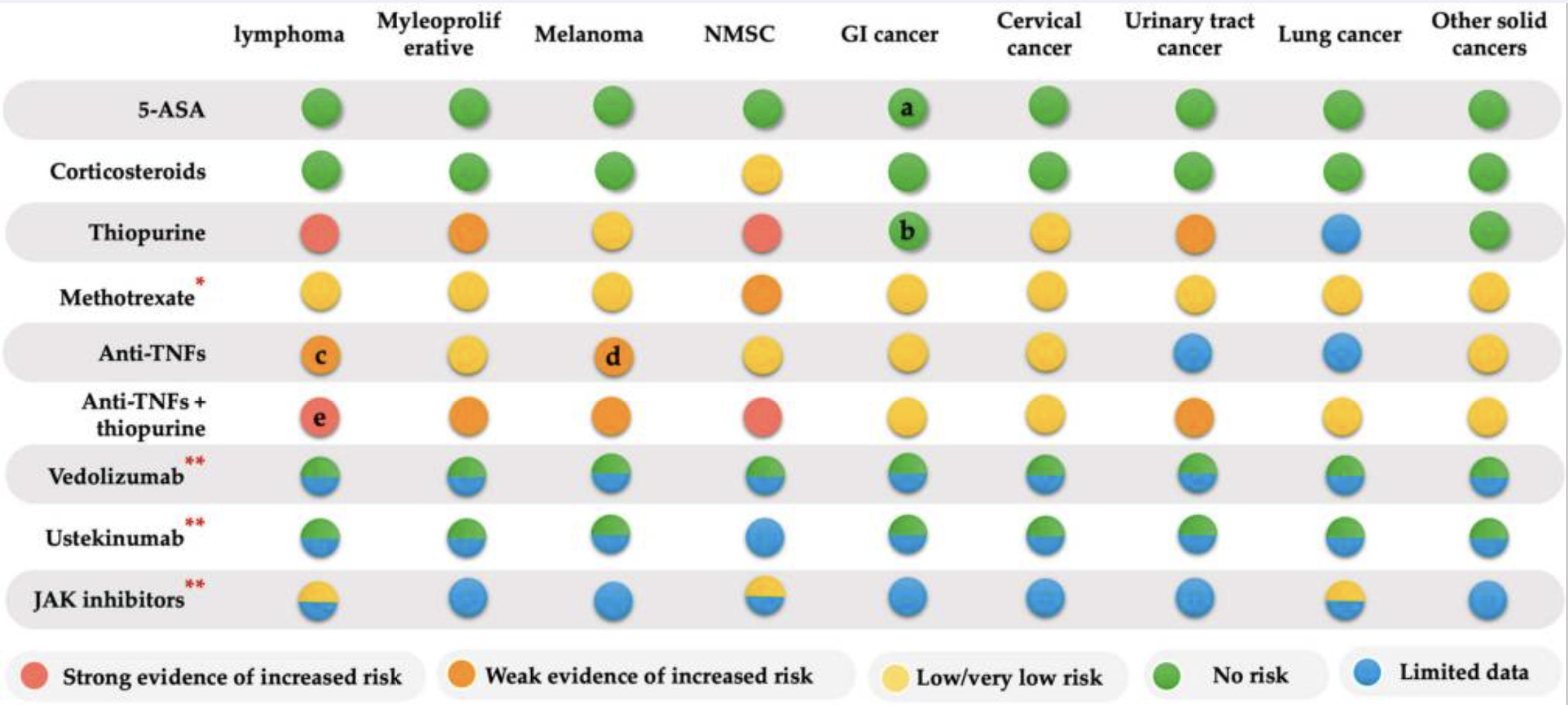
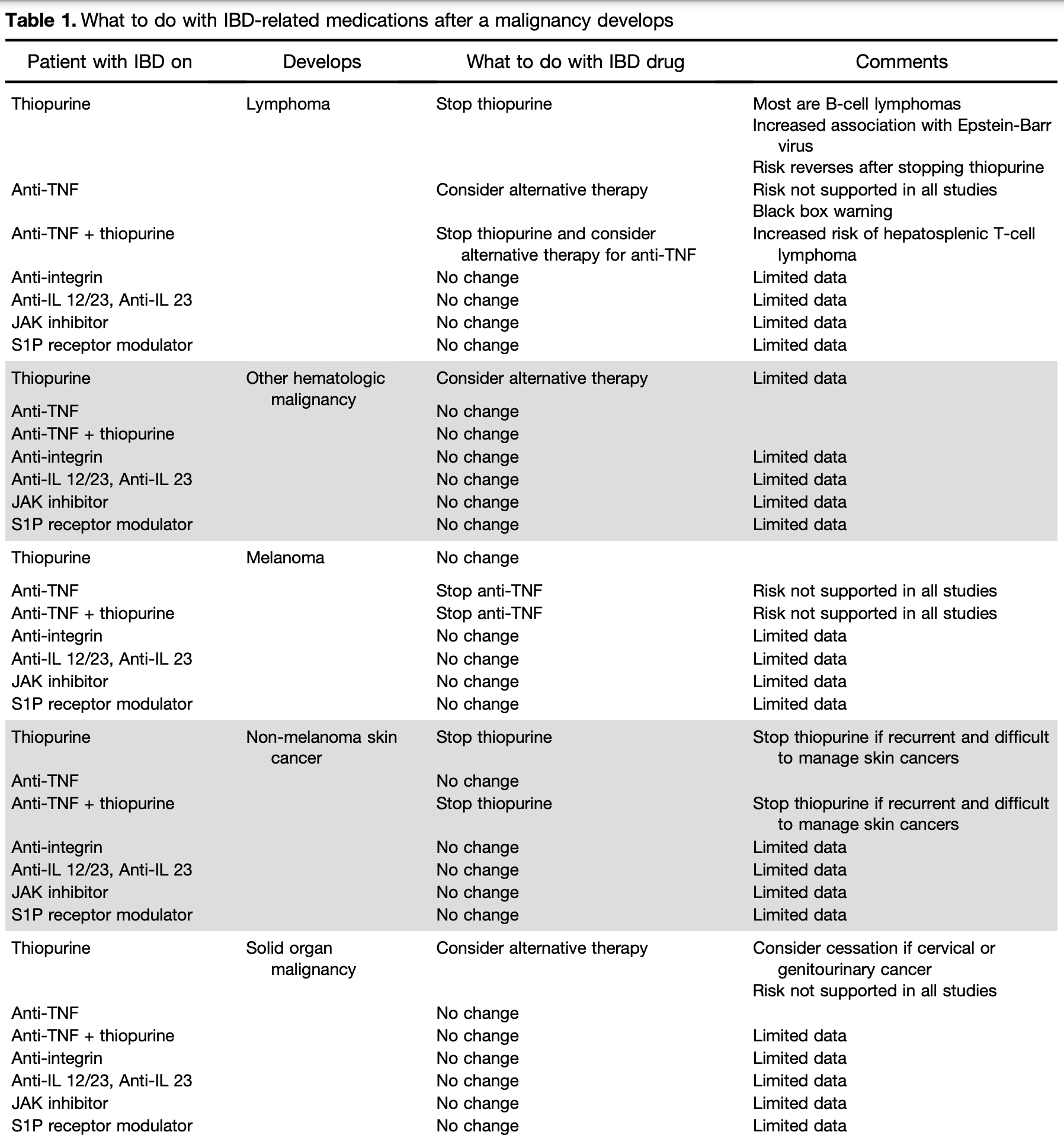
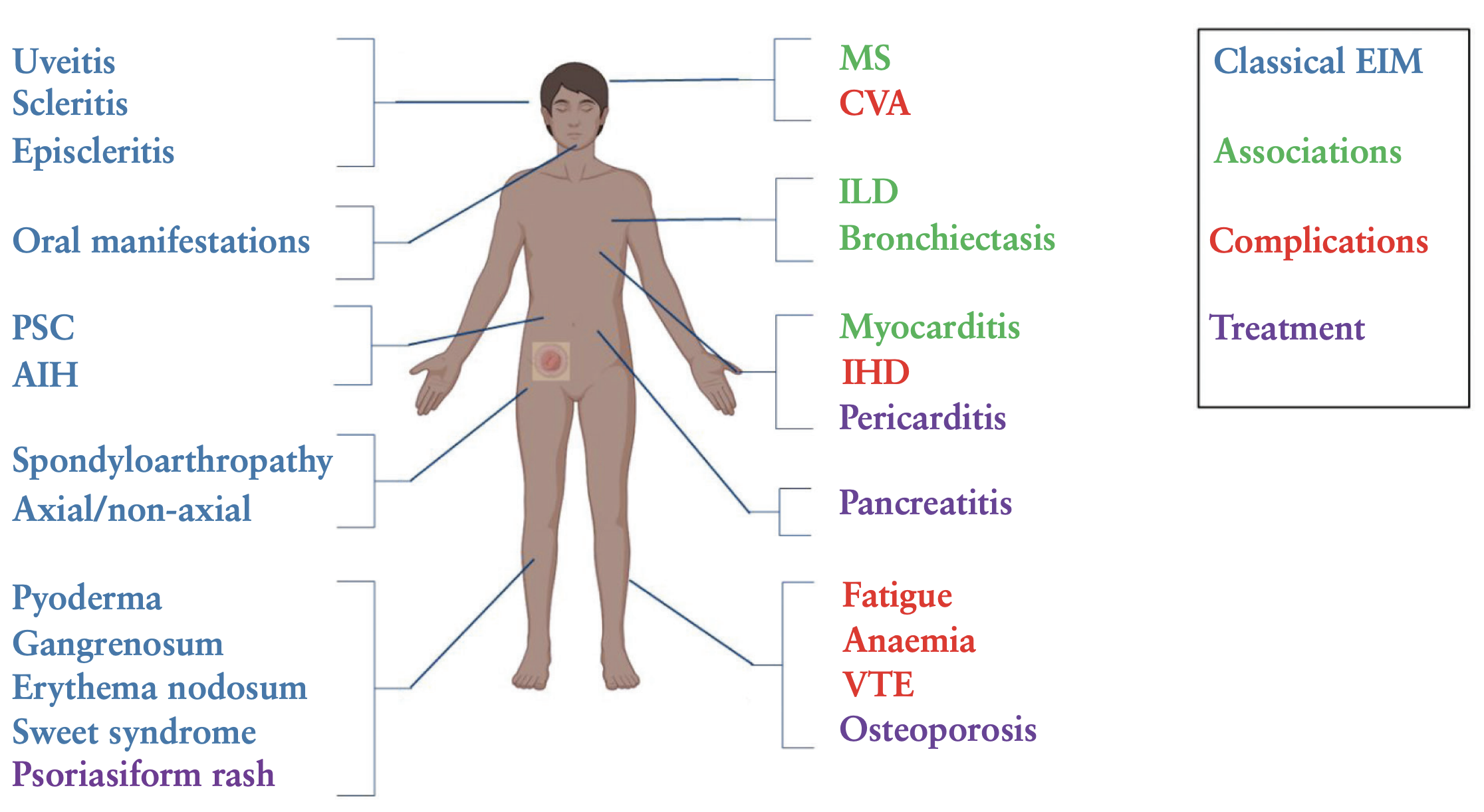
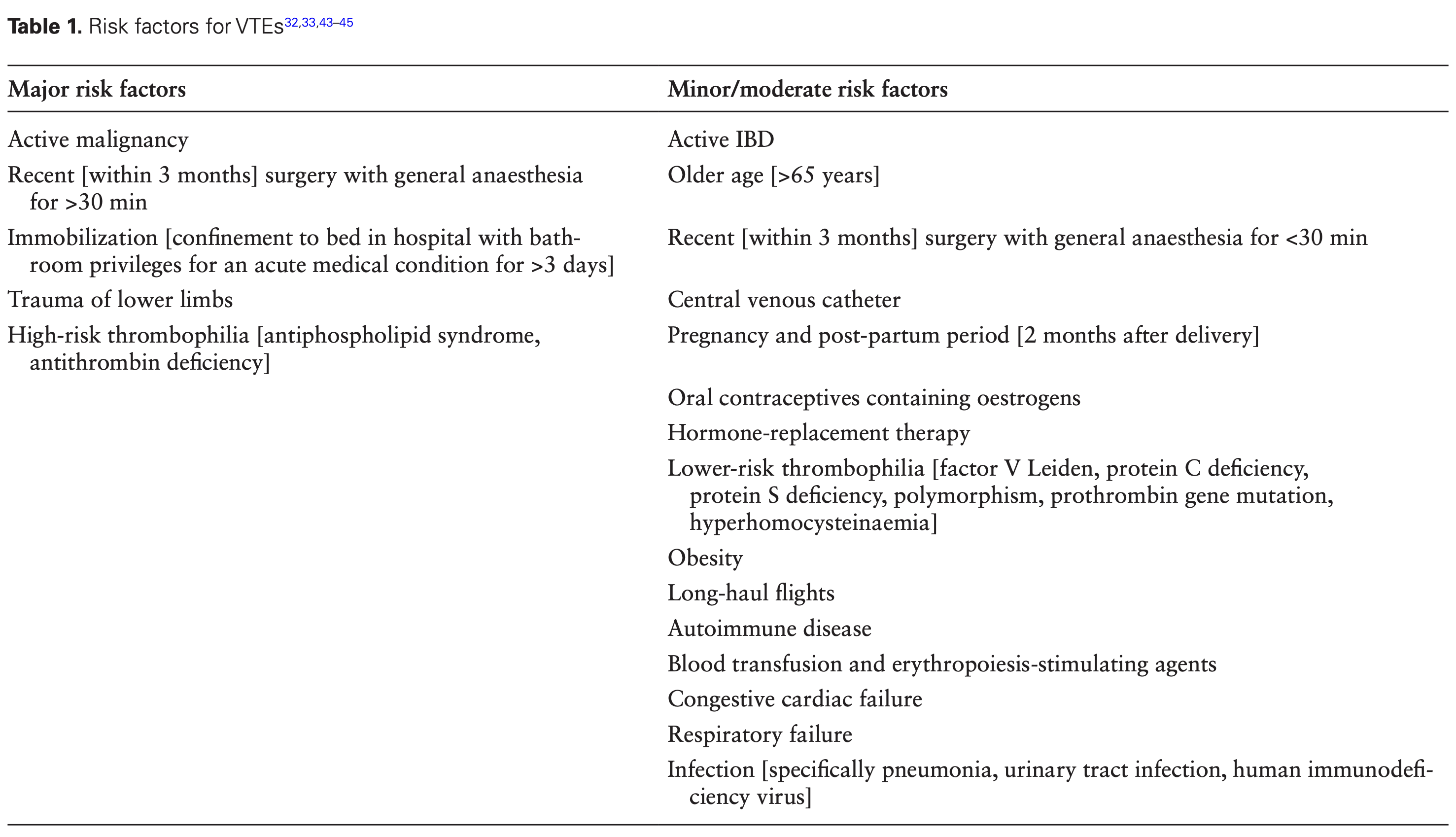
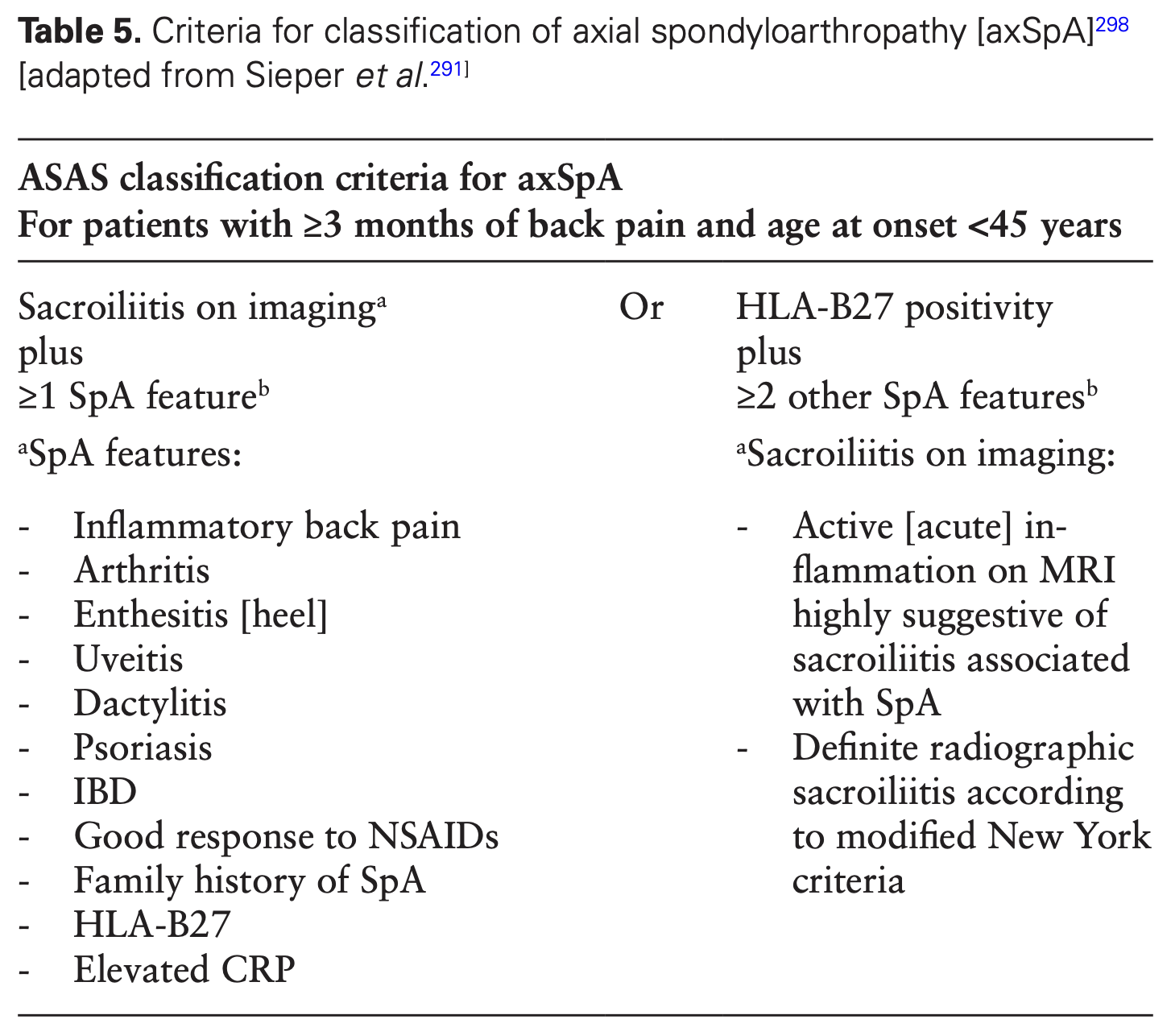
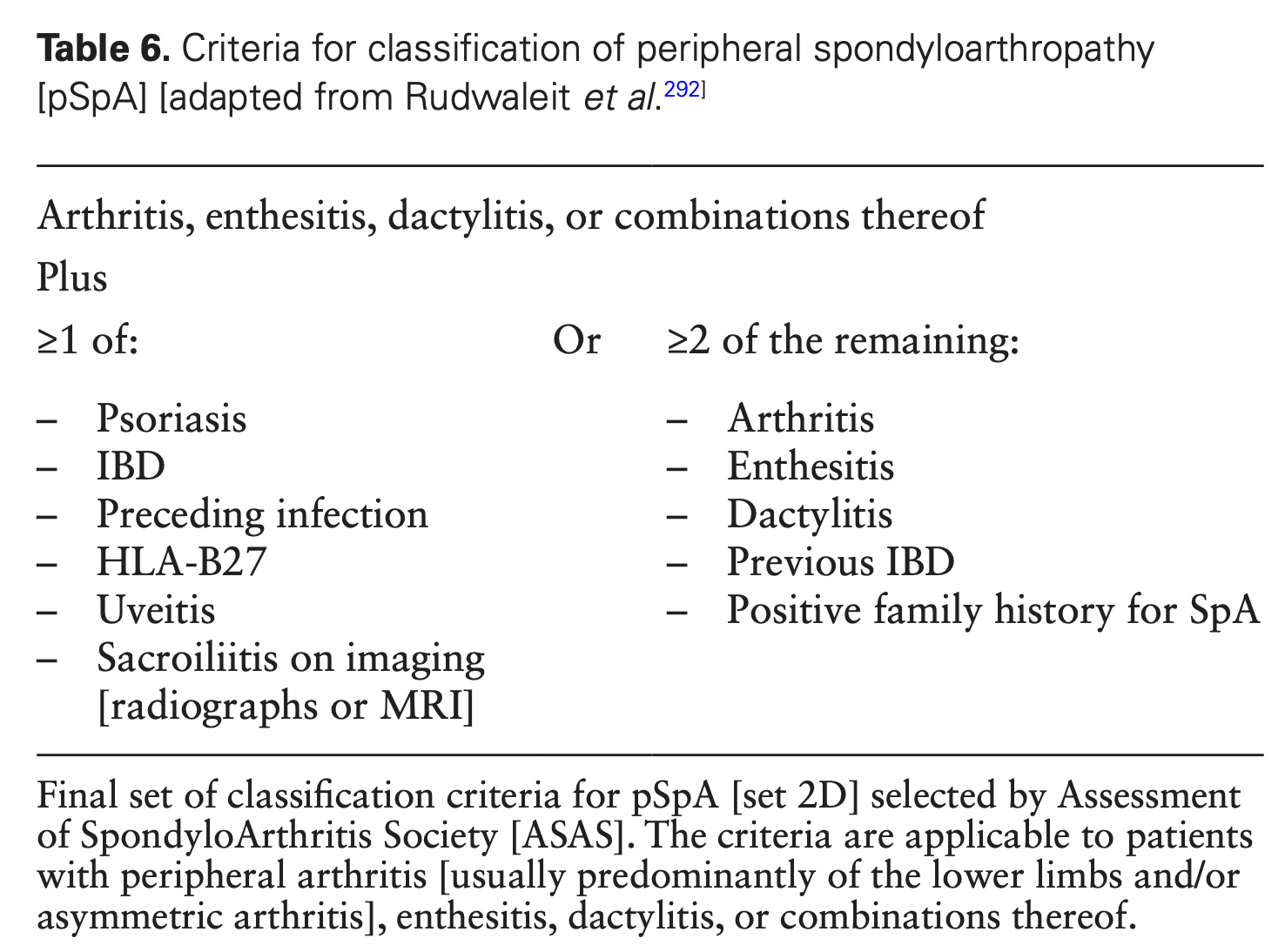
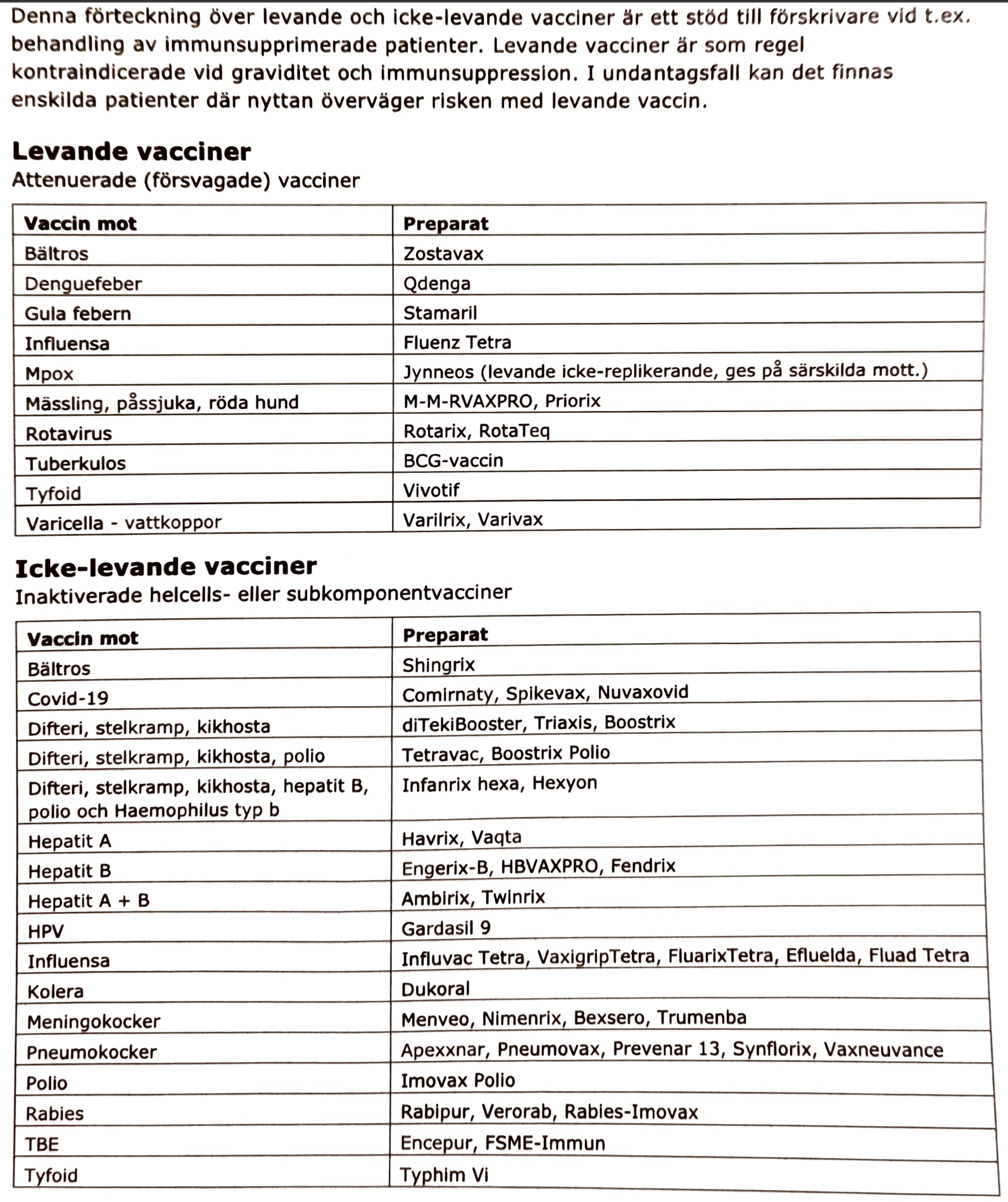
IBD
Crohn’s
Behandling
#Crohns behandling
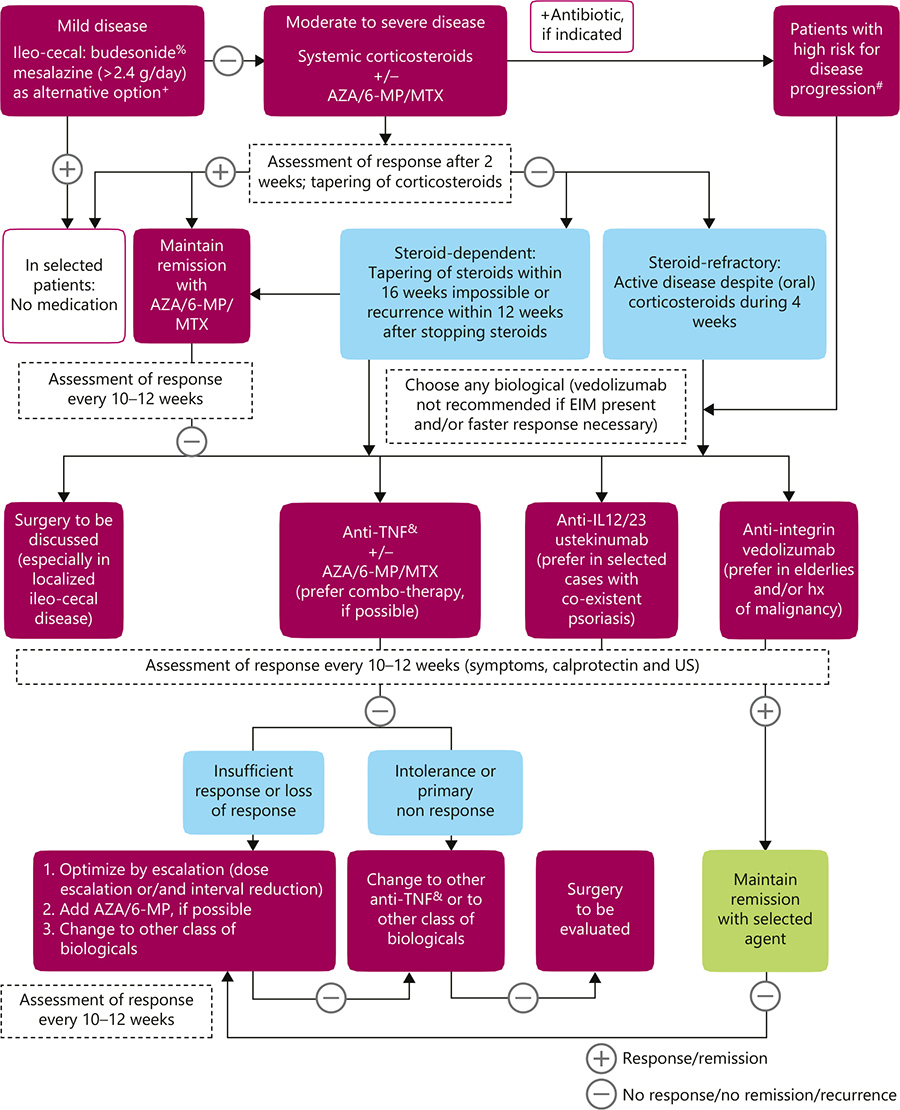
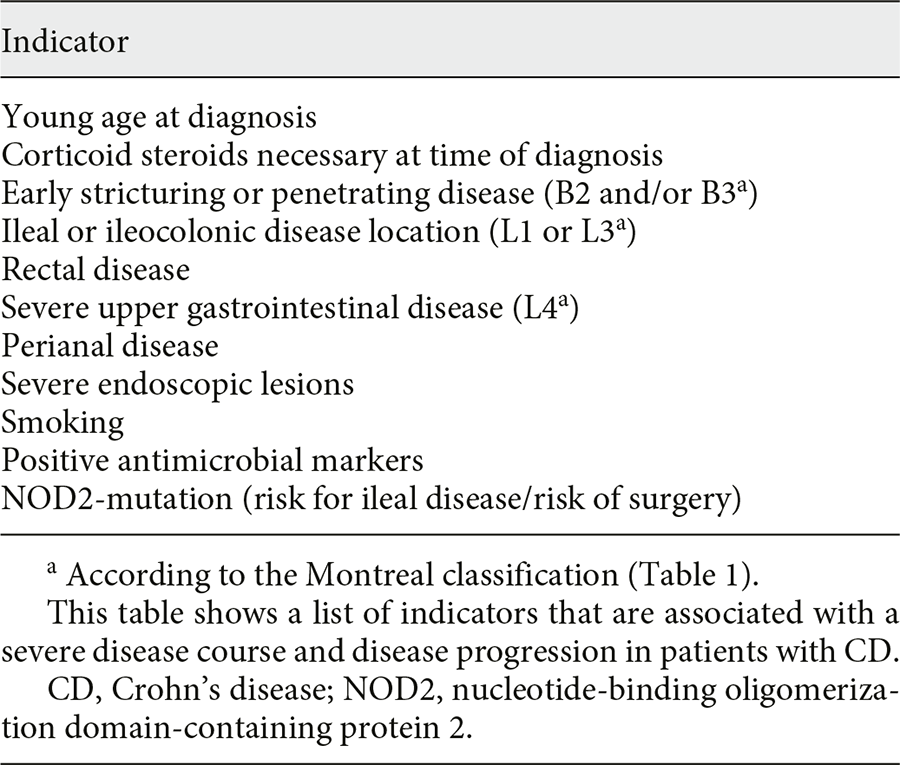
Sulz et al - Therapy algorithm for endoluminal CD. To keep this algorithm as simple as possible and for reasons of repetition and similarity, location and extent of inflammation (localized ileo-cecal, colonic, and extensive small bowel disease) are not specifically represented. Also, for reasons of simplicity, the severity of inflammation (mild/moderately severe/severe) is not differentiated. However, high-risk situation for progressive disease and steroid-dependent/steroid-refractory inflammation are discussed as separate entities. Surgery as a therapeutic option in endoluminal CD treatment is integrated in the algorithm, where indicated. + For more details, see main text. % Budesonide: cortiment multi matrix is not approved for treatment of CD. # Table 1: indicators for severe disease/disease progression. & Anti-TNF: infliximab and its biosimilars (Remicade®/Inflectra®/Remsima®); Adalimumab and its biosimilars (Humira®/Hyrimoz®/Amgevita®); CertolizumabPegol (Cimzia®), only approved in Switzerland and USA. CD, Crohn’s disease; AZA, azathioprine; IL, interleukin; 6-MP, 6-mercaptopurine; MTX, methotrexate; TNF, tumor necrosis factor; EIM, extraintestinal manifestations; US, ultrasonography.
Fistulerande sjukdom
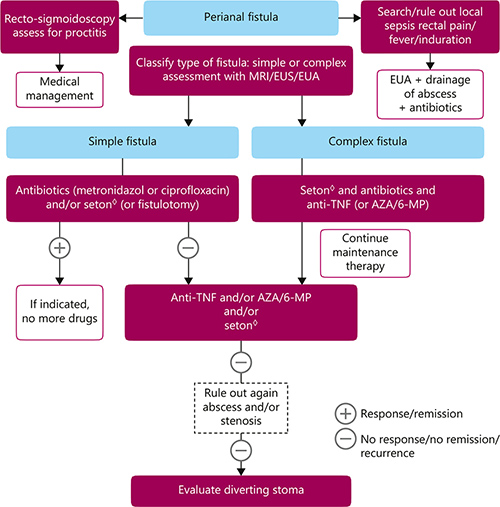
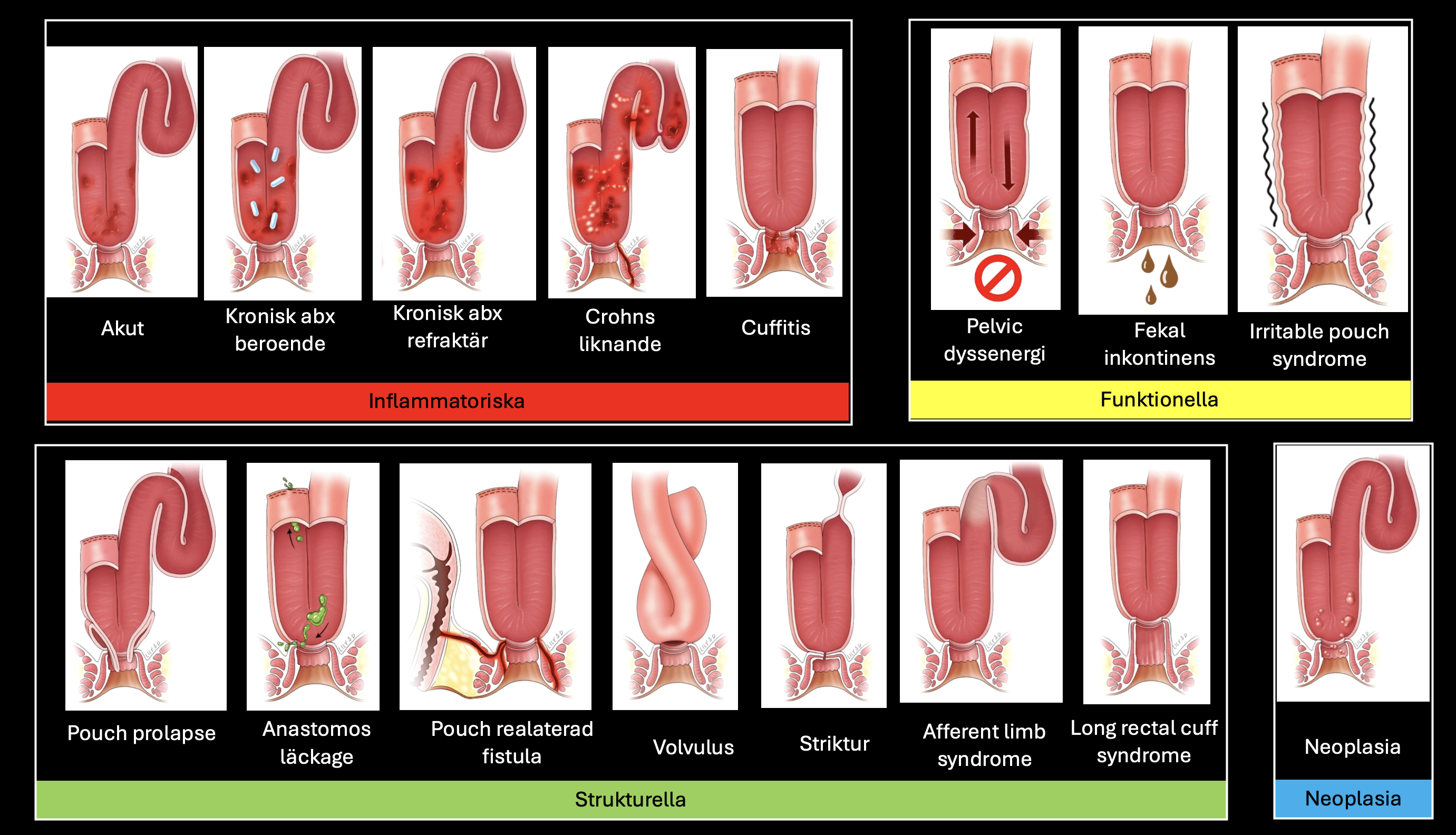
Sulz et al - Management of fistulizing perianal disease. Simple fistulae: Low fistulae that involve superficial tissue and include subcutaneous and intersphincteric and intrasphincteric fistulae that remain below the dentate line, have a single opening and are not associated with perianal complications. Complex fistulae: are high (high means involving >2/3 of the external sphincter) intersphincteric, high transsphincteric, suprasphincteric, and extrasphincteric, may have multiple openings, and may be associated with an abscess, proctitis, rectal stricture or may be connected with the bladder or vagina. ◊ Seton: Seton, not cutting. AZA, azathioprine; EUA, examination under anesthesia; EUS, endoscopic ultrasonography; 6-MP, 6-mercaptopurine; MRI, magnetic resonance imaging; TNF, tumor
Postoperative CD
#Post op Crohns
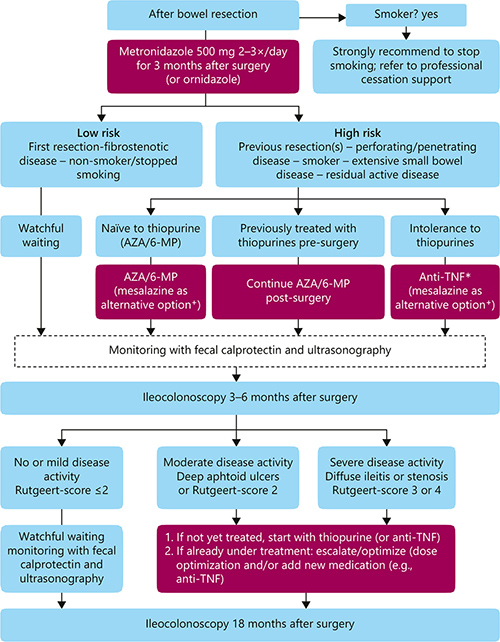
Sulz et al, Management of postoperative CD. #Rutgeert score #Crohn’s reccurence
Ulcerös kolit
Tiopuriner
Wartner et al: A practical guide to thiopurine prescribing and monitoring in IBD
#azathioprine #azatioprin #purinethol #purimmun #TPMT #6TGN
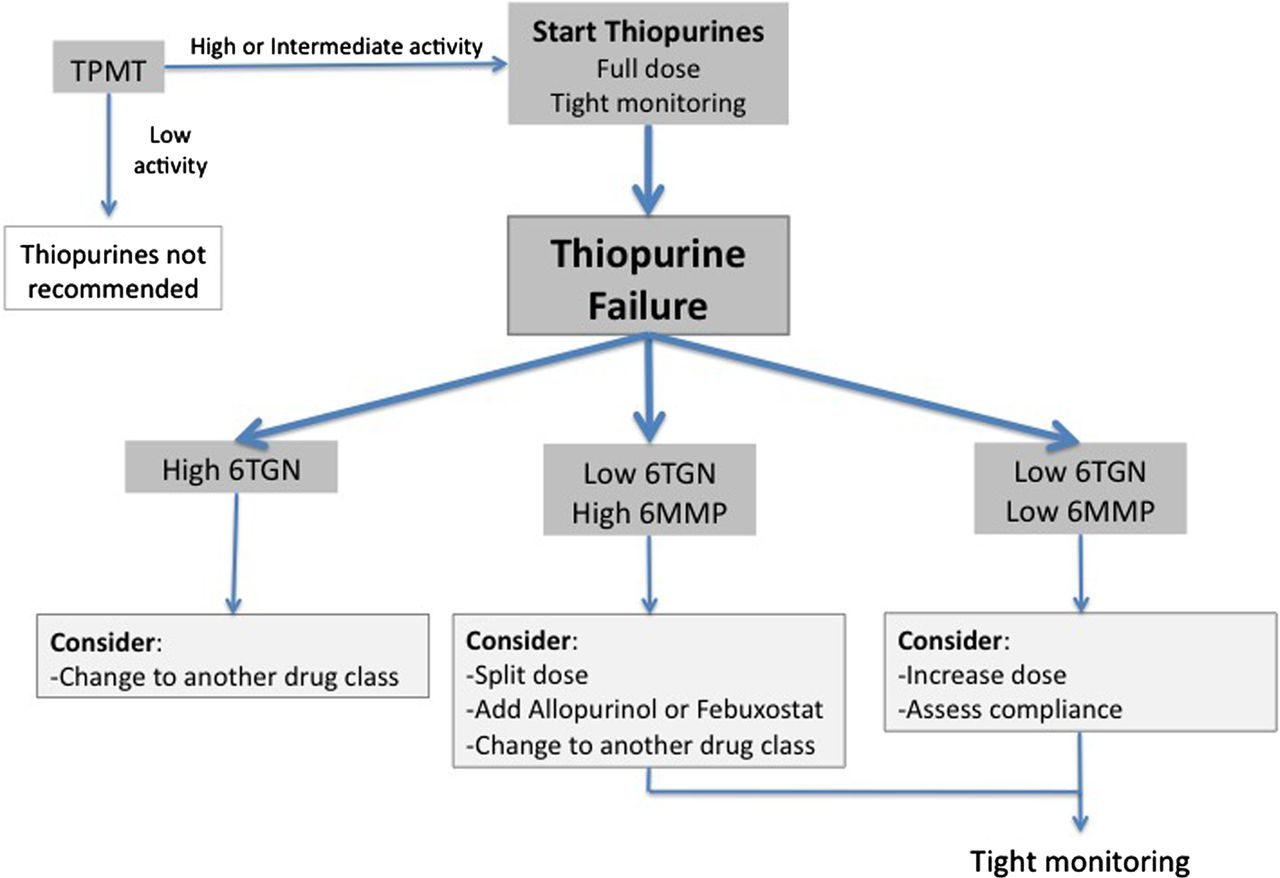

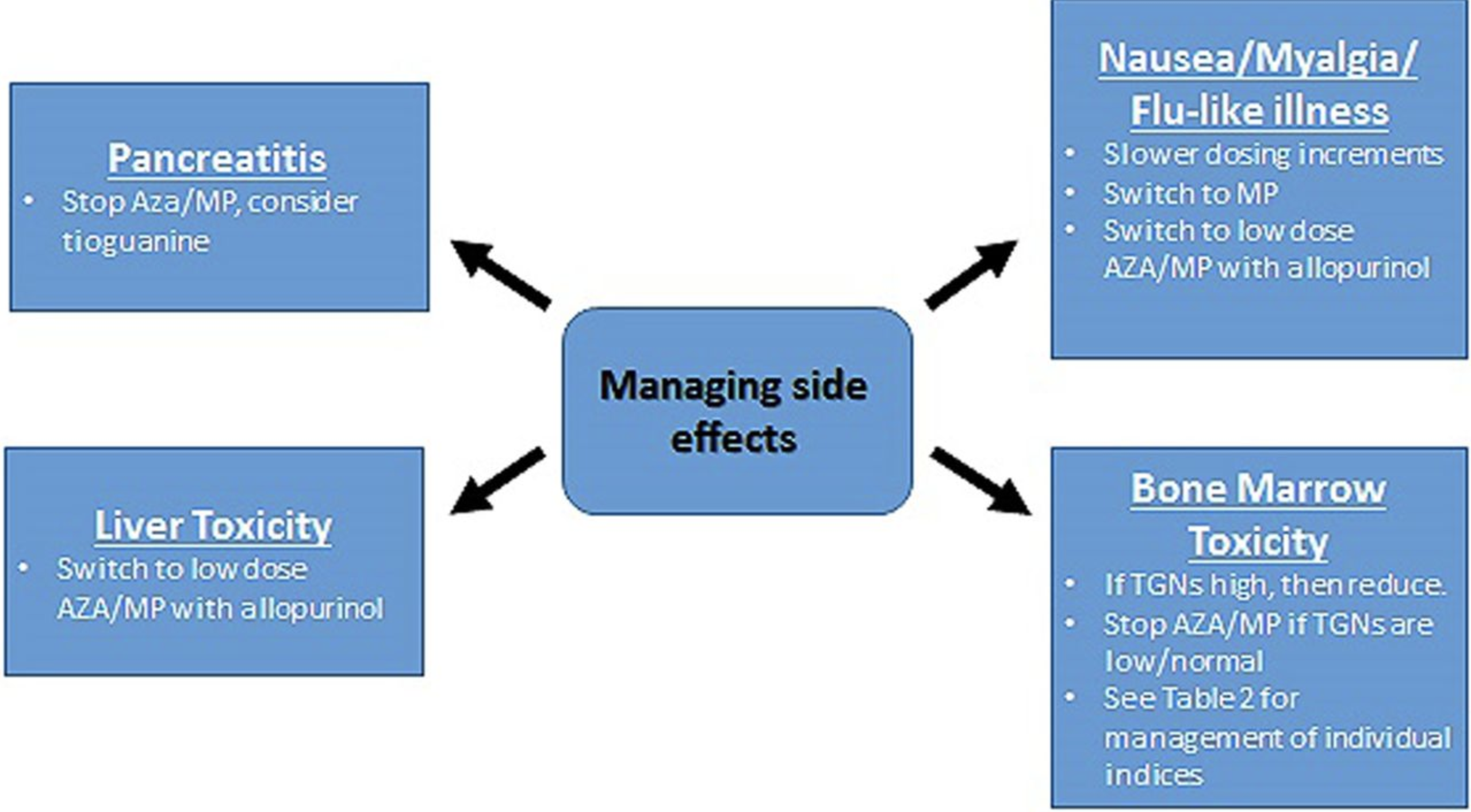
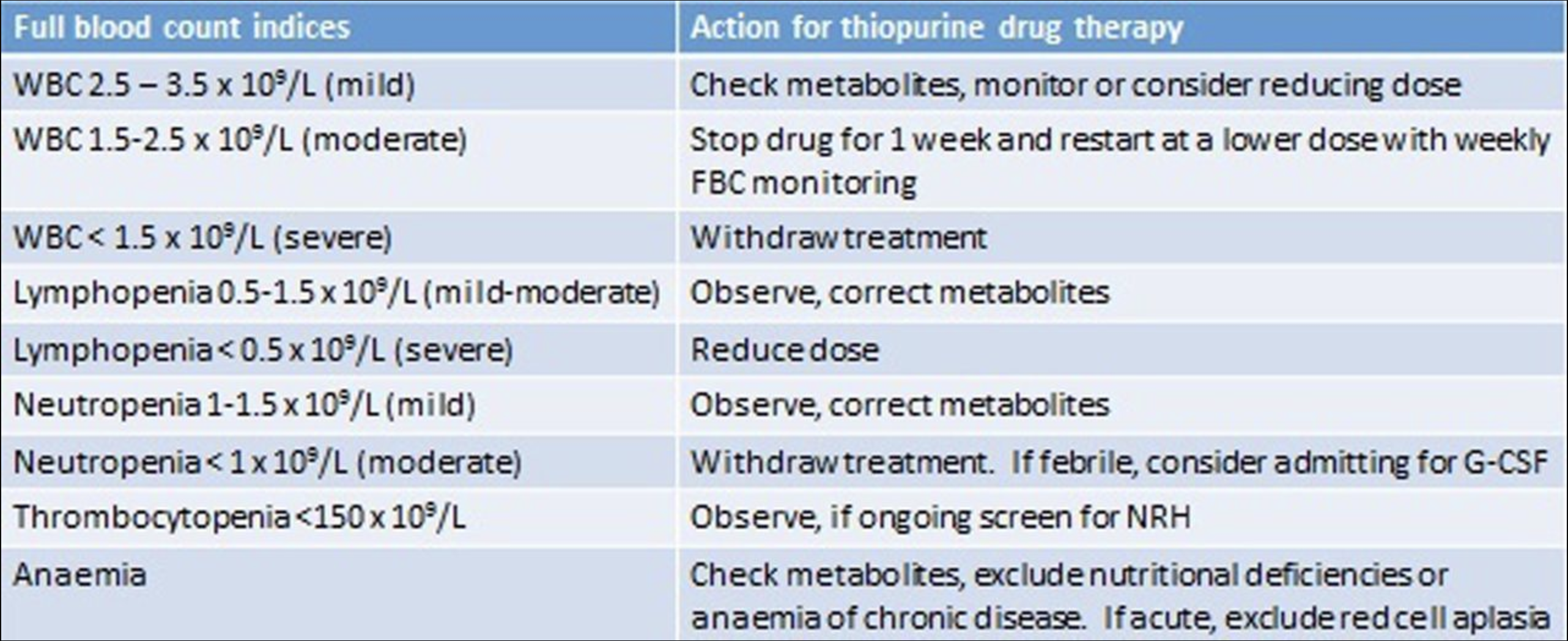
#Skev metabolisering